Instructions For Use
[General caution]
1)This product is for in-vitro diagnostic use only. Do not use for other purpose.
2)Diagnosis of Group A streptococcus infection should be made properly in conjunction with the assessment of clinical symptom and other test results.
3)Procedures that are not described in instructions for use are not guaranteed.
[Kit composition]
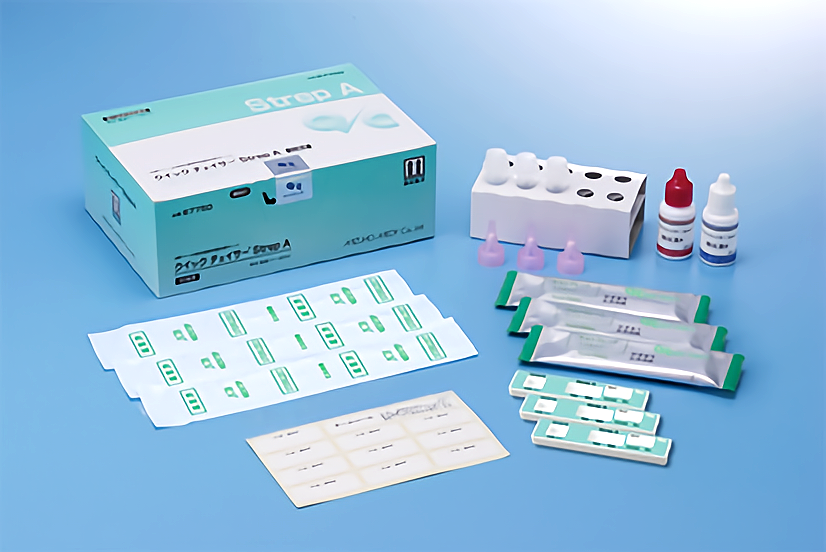
1)Test plate -10 tests
・Rabbit polyclonal anti-Group A streptococcus antibody
・Colloidal gold conjugated to rabbit polyclonal anti-Group A Streptococcus antibody
2)Extraction reagent A (2.0mol/L Sodium Nitrite) – 5mL×1
3)Extraction reagent B (0.2mol/L Acetic Acid) – 5mL×1
4)Sterile swab (For throat swab specimen) -10 pieces
5)Extraction reagent solution vial – 10 vials
6)Rack (for extraction reagent solution vial) – 1 piece
7)Filter (for extraction reagent solution vial) – 10 pieces
8)Name label (for extraction reagent solution vial) – 1 sheet
[Intended use]
For detection of Group A Streptococcus antigen in pharyngeal mucosa epithelial cell (To aid in the diagnosis of Group A Streptococcal infection)
[Principle of the test]
Quick Chaser Strep A is the in-vitro diagnostic reagent for detection of Group A Streptococcus antigen based on Immunochromatographic Assay.
Colloidal gold conjugated to rabbit polyclonal anti-Group A Streptococcus antibody and colloidal gold conjugated to mouse immunoglobulin for control line are coated in sensitized colloidal gold coating area in test plate. Rabbit polyclonal anti-Group A Streptococcus antibody is immobilized in the area of the test line
and anti-mouse immunoglobulin antibody is immobilized in the area of control line.
According to immunochromatographic principle, in the presence of Group A streptococcus antigen in sample, they migrate to the area between sample area and test line area, where reacting with colloidal gold conjugated to rabbit polyclonal anti-Group A Streptococcus antibody, and moreover, they react
with rabbit polyclonal anti-Group A Streptococcus antibody and are caught in the test line area, where visible purple-red line indicates the presence of Group A Streptococcus antigen.
Simultaneously, purple-red line is also visible for catching colloidal gold conjugated to mouse immunoglobulin on control line area, regardless of presence of Group A Streptococcus antigen.
[Procedural notes]
1)Do not use the collected specimen from other places than Pharynx area.
2)If a large amount of mucus (saliva, nasal secretion etc.) are included in specimen, it could give bad influence to reaction and cause incorrect test result. Therefore, be careful not to touch tongue,the inside surface of cheek and teeth for avoiding the collection of a large amount of mucus.
3)Collected specimen should be prepared as sample in accordance with proper method and should be tested as soon as possible.If the swab specimen cannot be tested immediately, keep the swab specimen in a clean and dry closed container and prepare the sample within 4 hours at room temperature or 48 hours at 2℃~ 8℃ for performing the test.
4)Keep volume of extraction reagent A and extraction reagent B (4 drops each). Proper result might not be obtained in case that the given amount is not kept.
5)Bring test plate and extraction reagent A and extraction reagent B to 15~30℃ prior to use.
6)Interfering substances and medications
Following substances and blood did not interfere the performance of this product at the concentration listed below.
・Cold medicine ① (Concentration of Acetaminophen: 5mg/mL)
・Cold medicine ② (Concentration of Ibuprofen: 5mg/mL)
・Gargle ①, containing Chlorhexidine gluconate (0.25%)
・Gargle ②, containing Myrrh Tincture (0.5%)
・Gargle ③, containing Povidoneiodine (3.25%)
・Oral antiphlogistic containing water-soluble azulene (10%)
・Cough drop ①, containing Dipotassium Glycyrrhizinate (20mg/mL)
・Cough drop ②, containing Dry Nandin Fruit Extract (10mg/mL)
・Cough drop ③, containing Cetylpyridinium chloride (20mg/mL)
・Acetylsalicylic acid (20 mg/mL)
・Diphenhydramine hydrochloride (5 mg/mL)
・Dextromethorphane (10 mg/mL)
・Blood (1%)
7)Cross Reactivity
・Bacteria
No cross reactivity was observed with Escherichia coli, Haemophilus in!uenzae, Klebsiella pneumoniae, Psedomonas aeruginosa, Candida albicans, Enterococcus faecalis, Staphylococcus epidermidis, Streptococcus pneumoniae,
Streptococcus mutans and Streptococcus sp. Group B,C,F,G
※No cross reactivity was observed with Staphylococcus aureus (1.0× 107 CFU /mL or lower)
・Influenza A virus
No cross reactivity was observed with A/PR/8/34(H1N1) and /Victoria/3/75(H3N2)
・Influenza B virus
No cross reactivity was observed with B/lee/40 and B/Mass/3/66
・Adeno virus
No cross reactivity was observed with Adenovirus type 1, 2, 3, 4, 5 and 6
・RSV
No cross reactivity was observed with A2, long, B1wild, 9320 and CH18537
[Procedure]
● Specimen collection
1)Preparation of specimen collection. Use sterile swab provided in this kit.
2) Specimen collection Insert sterile swab from oral cavity into pharynx. Collect
mucous epidermis by rubbing several times reddened area of a posterior wall of the pharynx or tonsil.
● Preparation of reagent
Bring test plate and extraction reagent A and extraction reagent B to 15~30℃ prior to use.
● Preparation of sample
● Details of test plate
[Test procedure]
1) Preparation of reagent
Test plate : No prior preparation required.
2)Test procedure
①Take out test plate from aluminum foil pouch.
②Apply 3 drops (About 140μL) of sample into the sample
area of test plate.
③Leave to react at 15℃~30℃.
④Interpret test results visually by lines in test line area and control line area after 3~5 minutes.
[Interpretation]
Interpretation by the existence of red-purple lines in test line area and control line area.
《Positive》Both test line and control line appear.
Positive
《Negative》Only control line appears.
Negative
《Retest》
Both test line and control line do not appear or no control line appears. Sample volume may not be enough. Recheck test procedure and retest with new test plate. If the same result come out in the retest again, confirm it with other method.
● Note
In case test line and control line appear at 3 ~ 5 minutes after applying sample, it can be interpreted as positive. Negative should be interpreted at 5 minutes after applying sample. Streak line might appear before 5 minutes temporarily due to flow of colloidal gold. Do not interpret the temporal streak line as appearance of
test line. Moreover, after several hours, colloidal gold could appear like line due to drying of test plate with time. Therefore, please interpret test results at 5 minutes.
[Performance]
1)Performance
①Sensitivity
・When in-house positive controls(※1) are tested, positive results are obtained.
②Accuracy
・When in-house positive controls are tested, positive results are obtained.
・When in-house negative controls(※2) are tested, negative results are obtained.
③Reproducibility
・When in-house positive controls are tested three times simultaneously, positive results are obtained in all cases
・When in-house negative controls are tested three times simultaneously, negative results are obtained in all cases.
※1 Reference Group A Streptococcal antigen solution diluted by extraction reagent A and B to 5.0× 10 5 CFU/mL.
※2 Buffer diluted by extraction reagent A and B
④Detection limit 1.0×10 5 CFU/mL
2)Correlation
Comparison with existing immunochromatographic test product approved in Japan
Negative agreement rate:100%
Positive agreement rate:100%
Total agreement rate:100%
Negative agreement rate:100%
Positive agreement rate:96.4%
Total agreement rate:98.1%
※ 2 discrepant specimen showed positive by PCR and other product (1)
3)Calibration standard : ATCC19615
[Precaution for use, handling]
1)Precaution for handling (Prevention of danger)
①HIV, HBV, and HCV could be included in sample (specimen).
Be careful of handling specimen as potentially infectious material.
②Be careful not to touch sample (specimen) or extraction reagent solution directly to skin or not to get them into eyes.
③Do not use swab to collect specimen, if it is already put into extraction reagent solution.
④If sample (specimen) and/or extraction reagent solution are accidentally got into eyes or mouth, flush with plenty of water as emergency treatment, and see a doctor if necessary.
⑤2.0 mol/L Sodium nitrite is contained in Extraction reagent A and 0.2 mol/L Acetic acid is contained in Extraction reagent B so they are accidentally got into eyes or mouth, flush with plenty of water as emergency treatment, and see a doctor if necessary.
⑥Do specimen collection under guidance of technically qualified person.
⑦Raw material of membrane used in test plate is nitrocellulose.
Do not perform test near fire because nitrocellulose is extremely flammable material.
⑧Wipe scattered sample (specimen) off by alcohol for disinfection.
2)Precaution for use
①Do not freeze. Store in accordance with description of instruction for use. Do not use frozen reagents because they could show incorrect test result.
②Do not use beyond expiration date.
③Immediately use test plate after opening of foil pouch. If it is left in air for long time after the opening, it could be non-reactive due to getting moistened.
④Do not touch sample area, test line area and control line area by hands directly.
⑤Do not perform test in the place such as under air conditioner where the dry wind blows the test plate into uneven migration.
⑥Even though patients are infected by Group A Streptococcus , test result can be negative because Group A Streptococcus antigen in the specimen may be below the detection limit of the test. Moreover, negative specimen could be interpreted as positve by non-specific reaction due to some factors in specimen. Diagnosis of Group A streptococcus infection must be evaluated in conjunction with results of other tests and clinical symptom.
⑦Do not use reagent, accessaries etc. of the product except the purpose of the test.
⑧Test plate, swab, and extraction reagent solution vial (including filter) are intended for single use only.
⑨Use only swab included in the kit.
⑩Do not touch spherical tip before use.
⑪Use swab immediately after opening package.
⑫If break and/or hole are found on the package of swab, do not use it because it is not in sterile condition.
⑬If swab is stained, broken or bent, do not use it.
⑭Do not bend the rod of swab before collecting specimen.
⑮Be careful not to injure region to be collected (mucosa) and break the rod of swab by pushing too hard at the time of collecting specimen by swab.
⑯Be careful not to splatter the sample at the time of taking the swab out of vial after preparing sample.
⑰In case collection volume of specimen is excess, or high-viscosity, membrane filter could be clogged and adequate sample volume could not be dropped. In such cases, collect specimen again and retest it with new test plate.
⑱Do not mix each kit composition from different lot numbers.
⑲Be careful not to interchange bottle caps with extraction reagents because it could make extraction reagents mix together
3)Precaution for waste disposal
①Treat liquid waste and used utensils by any one of following methods because specimen could contain infectious material such as HIV, HBV, and HCV etc.
a) Immerse in Sodium Hypochlorite solution (Valid chlorine concentration 1000ppm) for more than 1 hour.
b) Immerse in 2% glutaraldehyde solutions for more than 1 hour.
c) Autoclave at 121℃ for more than 20 minutes.
②Regarding disposal of reagents and utensils, dispose of them in accordance with local law and regulation.
[Storage・Expiry]
・Storage: 1 ~ 30℃
・Expiry: 24 months (As indicated on package)
[Reference]
1)Lancefield,R.C.:J.Exp.Med.,57;571-591,1933